The Using Patient Preference Information in the Design of Clinical Trials (PPI-CT) Framework is focused on providing medical device clinical trial sponsors and the broader clinical trial community an actionable set of learnings and compendium of best practice...
Search Results

Quantifying Benefit-Risk Preferences for Heart Failure Devices: A Stated-Preference Study
This published article summarizes the work of a multi-stakeholder group of experts convened by MDIC to develop and conduct a patient preference study among patients living with heart failure. The goal of this effort was to gain a better understanding of attributes for...

Patient-Centered Identification of Meaningful Regulatory Endpoints for Medical Devices to Treat Parkinson’s Disease (PCOR Project Aim 1 Paper)
A growing body of literature has developed identifying outcomes that matter to patients. The study that these published articles (Aims 1, 2 & 3 of MDIC’s PCOR project) are based on demonstrates an approach to identifying outcomes of medical devices for Parkinson’s...

Parkinson’s Patients’ Tolerance for Risk and Willingness to Wait for Potential Benefits of Novel Neurostimulation Devices: A Patient-Centered Threshold Technique Study (PCOR Project Aim 2 paper)
A growing literature has developed identifying outcomes that matter to patients. The study that these published articles (Aims 1, 2 & 3 of MDIC’s PCOR project) are based on demonstrates an approach to identifying outcomes of medical devices for Parkinson’s disease...

Patient-Centered Clinical Trial Design Tool for Heart Failure Devices Methods Report
Clinical trials are designed based on a statistical approach to gain confidence that a product is safe and effective. However, traditionally, these statistical considerations have not quantitatively incorporated patients perspectives on benefit-risk tradeofffs. For...
Video Release for Patient Preferences for Heart Failure: A Collaborative Study
Through this project, MDIC brought together six heart failure device companies to work in collaboration to develop an understanding of what matters to patients living with heart failure, especially how those patients might be willing to accept potential risks from...

Incorporating Patient Preferences in Clinical Trial Design and Research
This whitepaper was authored by Margaret Sheehan and Anne Cohn Donnelly, who live with Parkinson’s disease and have participated as Patient Scientists on the MDIC PCOR project team along with representatives from the Michael J. Fox Foundation for Parkinson’s Research...
Case Study: Using the MDIC Patient Centered Benefit-Risk Framework to Support an Expanded Indication
Some patients may require dialysis if their own bodies are unable to filter toxins from their blood. Hemodialysis is a type of dialysis in which blood is pumped from the body through a filter and then back into the body. Typically, in-clinic treatment requires...
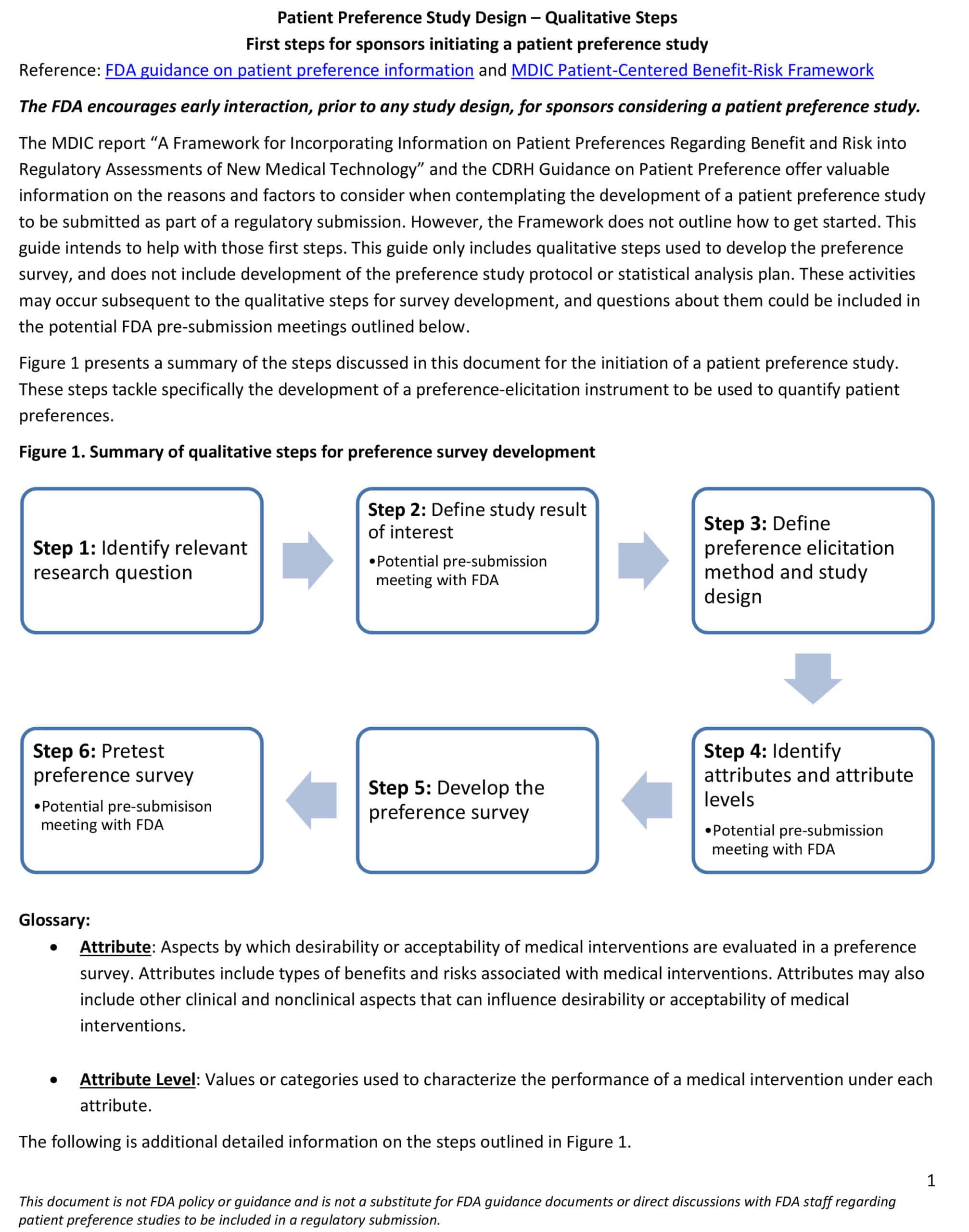
Patient Preference Study Design Guide – Qualitative Steps: First steps for sponsors initiating a patient preference study
This study design guide provides an outline of the qualitative steps for initiating a patient preference study. The guide only includes qualitative steps used to develop the preference survey and does not include the development of the preference study protocol or...

A Framework for Incorporating Information on Patient Preferences Regarding Benefit and Risk into Regulatory Assessments of New Medical Technology
The Patient Centered Benefit-Risk (PCBR) Framework introduces the concept of the total product development lifecycle and discusses how patient preference information might be useful at each stage of this lifecycle. The report provides background on the concepts of...
