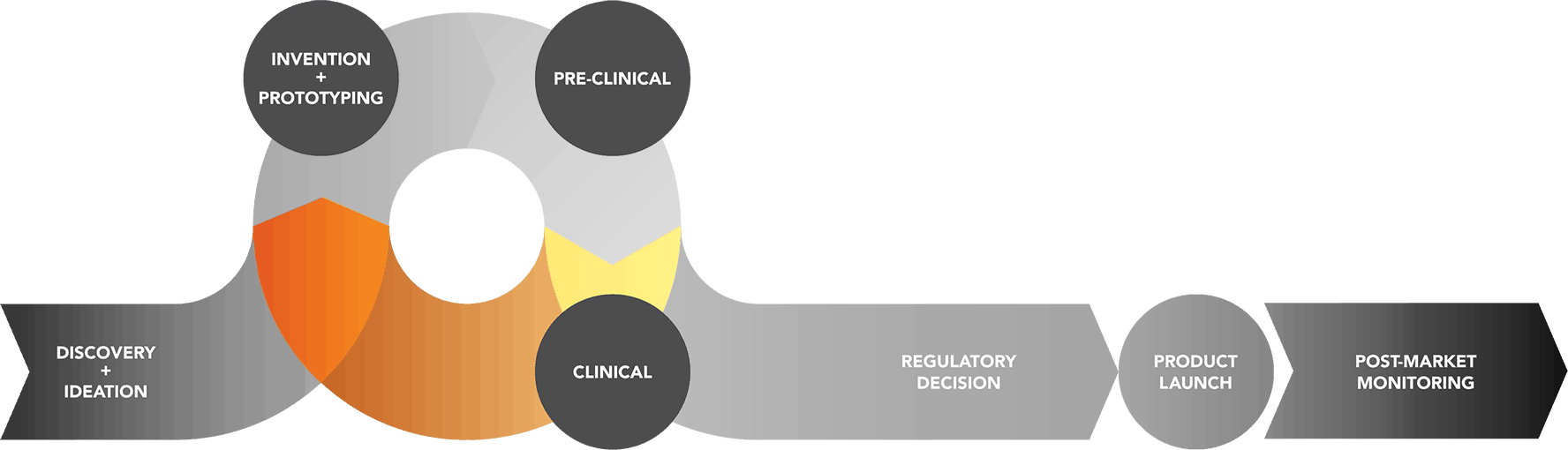
Total Product Life Cycle : Clinical Phase
The term “clinical research” refers to studies, or trials, in which the device is tested in humans. Once a sponsor has sufficient pre-clinical data, the next step is to plan for clinical studies to establish the safety and effectiveness of the product. The Federal Food, Drug, and Cosmetic Act established a risk-based device classification system for medical devices. Each device is assigned to one of three regulatory classes Class I, Class II or Class III, based on the level of control necessary to provide reasonable assurance of its safety and effectiveness and the level of regulatory control. Read about the Total Product Life Cycle here »
Articles in
