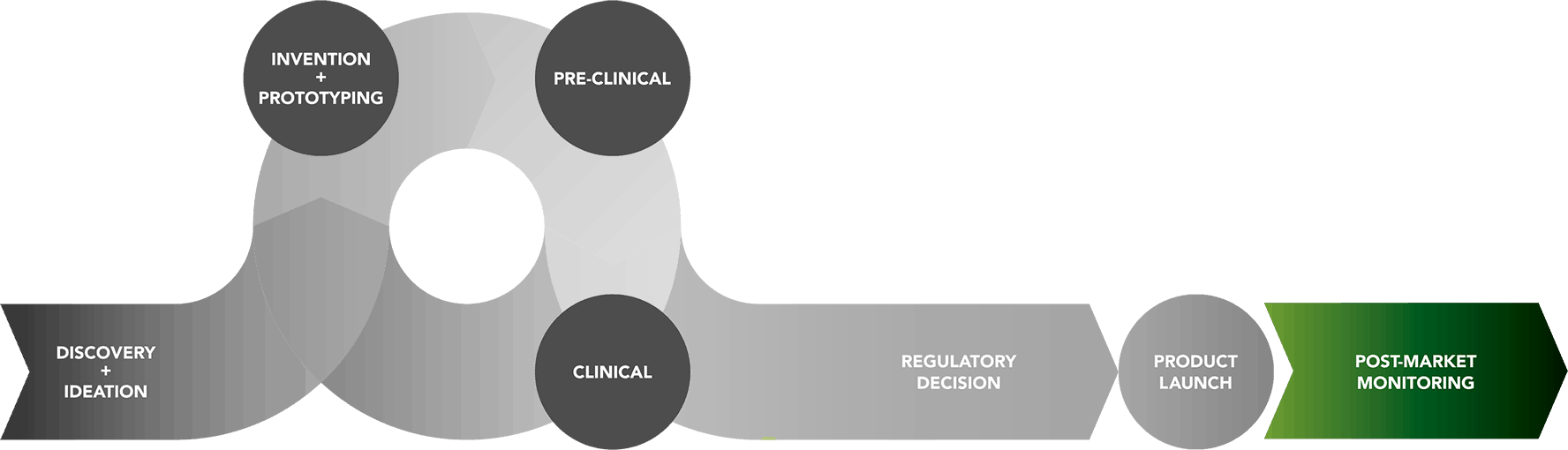
Total Product Life Cycle : Post-Market Monitoring Phase
Even after granting marketing authorization for a medical device, the FDA continues to monitor how it performs once it is commercially available. Although pre-market clinical trials provide important information on a device’s safety and effectiveness, it is possible that new safety concerns will emerge once the device is widely used by people. FDA has several programs that allow manufacturers, health professionals, and consumers to report problems associated with approved medical devices to make sure that any safety issues are identified and can be addressed. This ongoing, post-marketing surveillance is critically important to ensuring patient safety. Read about the Total Product Life Cycle here »