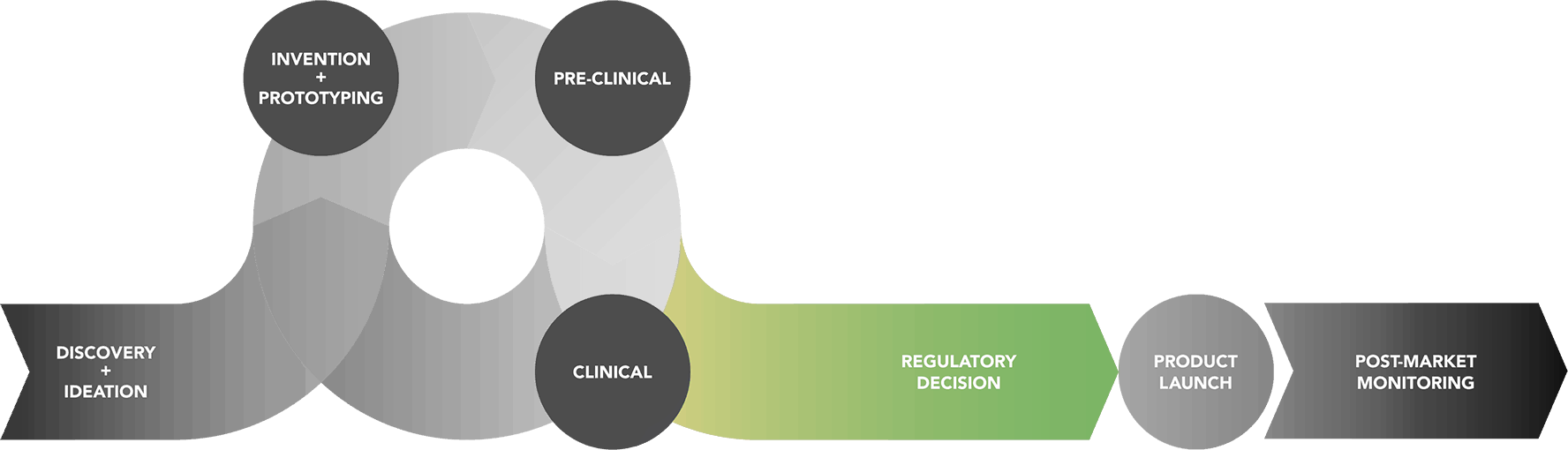
Total Product Life Cycle : Regulatory Decision Phase
If the clinical studies yield sufficient information on a device’s safety and effectiveness, the sponsor can submit an application to FDA to market the device to the public. There are several types of applications, depending on which class of device is being developed.
If appropriate, FDA can consult an Advisory Committee at a public meeting. FDA Advisory Committees consist of groups of experts who provide FDA with independent advice on whether a product should be approved or not. After the Advisory Committee meets, FDA decides about the approvability of the device or request additional information. By law, FDA must publish its decision with all supporting evidence in the Federal Register.
Read about the Total Product Life Cycle here »